Lifecare achieved the two major milestones for 1H2022 in June and continue to strengthen the organization.
It was a very important milestone for Lifecare to conclude successful, high-quality and reproduceable in-vitro test results with the fabricated Sencell sensor prototypes for the clinical trials on 2 June 2022: https://newsweb.oslobors.no/message/563975
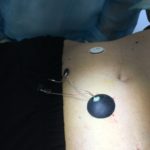
Following this achievement, Lifecare continued the prototype manufacturing and preparations for clinical trials, including insertion of the wired sensor in the tip of a needle and sterilization of the device. Theoretical studies implied good reason to expect that the chosen method of sterilization would be effective without interfering with the Sencell technology. Indeed, the selected sterilization method was successful without interfering with the quality of neither electronics, chemical fluid nor sealing of the sensors. On this basis Lifecare announced the start of the first-in-human clinical trials on 23 June 2022:
https://newsweb.oslobors.no/message/565607
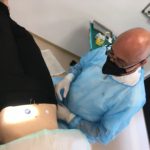
The clinical study was initiated as announced on June 23 and will continue until end of September. In the process leading towards initiation of clinical trials, the Lifecare organization has proven to be able to adopt, develop and execute a range of complex interdisciplinary tasks and manufacturing challenges over short timeframes.
To answer questions and requests for update from Lifecare shareholders, we want to underline that a clinical study as now is being carried out, is a complex operation. The technology and device have never been used by humans.
The purpose of this first study is to provide proof of concept confirmation for the measurement of glucose in humans and to further improve the device specifications in light of the upcoming later clinical studies for CE approval. Lifecare will prioritize to carry out the study with a high degree of quality. Furthermore, we will prioritize to spend the necessary time to ensure and interpret results forming a base for solid conclusions on the study results before we will communicate any conclusions or outcome.
The first patient has tested Sencell, and on this base Lifecares internal Clinical Evaluation Committee consisting of Lifecares CSO Prof. Andreas Pfützner, Board members Prof. Lutz Heinemann and Dr. Bo Petersson states to be encouraged to move forward with the clinical study as planned.
Lifecare will not comment further on the clinical study until conclusive data is achieved from a reasonable number of study participants.
Increasing the staff in the Lifecare Group
Lifecare continue to increase the staff at the headquarter in Bergen as we have strengthened the team with an Administrative Coordinator starting in July and a Communications & Public Relations Manager who will join the team latest 1 October.
As a result of the current milestone achievements Lifecare plan to increase the capacities in our cooperation with the University of Bath lead by Prof. Tony James. We are evaluating how to further strengthen the cooperation and Lifecares on-site activities in Bath, to ensure that Lifecare is positioned to initiate efficient developments to facilitate any requests for monitoring other analytes than glucose for patients with diabetes. As the first step in this direction, we are looking for a PhD student to join the team from October 2022: https://www.findaphd.com/phds/project/chemical-sensors-for-analytes-of-biological-importance/?p145702
Semi-annual report
Lifecares semi-annual report for 1H2022 will be published 25 August 2022 and the company plan to present the report in a webcast the same day. Invitation and link to the webcast will be announced on Oslo Børs Newsweb prior to 25 August 2022.
Until then we wish all our shareholders, partners, and other stakeholders a good summer!