Press releases
Lifecare – Sanofi Product Development Agreement Update
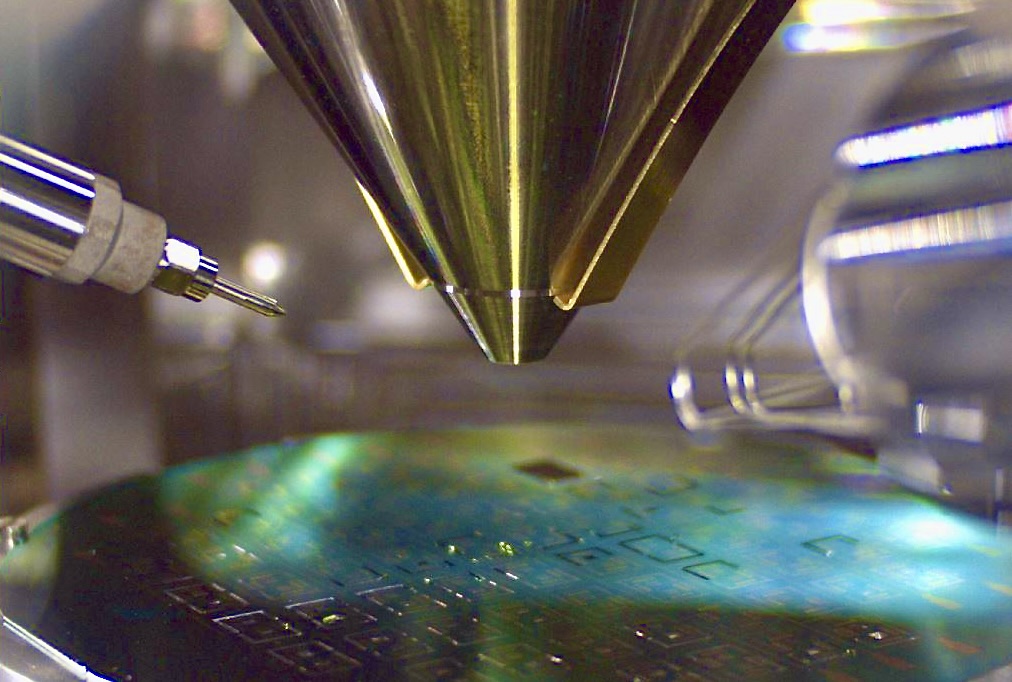
Bergen, Norway, 29 October 2024: Lifecare ASA (LIFE), a clinical stage medical sensor company developing the next generation Continuous Glucose Monitor (CGM), announces the finalization of a new development phase under the Company’s Product Development Agreement with Sanofi.
Reference is made to previous investor communication, dated 23 September 2021 and subsequent updates. Lifecare is engaged in a Product Development Agreement to advance the miniaturization of Lifecare’s sensor technology for integration into Lifecare’s Sencell Continuous Glucose Monitoring system.
Under the terms of the agreement, Sanofi has committed to providing financial contributions to support further development of Lifecare’s technology. In return, Lifecare has granted Sanofi a right of first refusal to negotiate a global and exclusive license (for human application) to Lifecares patented and proprietary glucose monitoring technology.
Upon completion of a defined development phase, Lifecare will today submit a phase-end report to Sanofi. This event triggers the release of funding contribution, while the Product Development Agreement continues in accordance with the development progress. This phase-end report does not directly activate commercial rights, but it highlights Lifecare’s commercial progress, demonstrating the achievement of previously reported technological milestones.
Subject to the continued development progress, Lifecare expects to submit the next phase-end report under the Product Development Agreement after completing its longevity study in dogs, targeted for Q1 2025. This report could potentially activate commercial rights, however, Lifecare has no indication at this stage as to whether these commercial rights will be triggered.