Information from Lifecare – August 2022
Posted: 08/08/2022
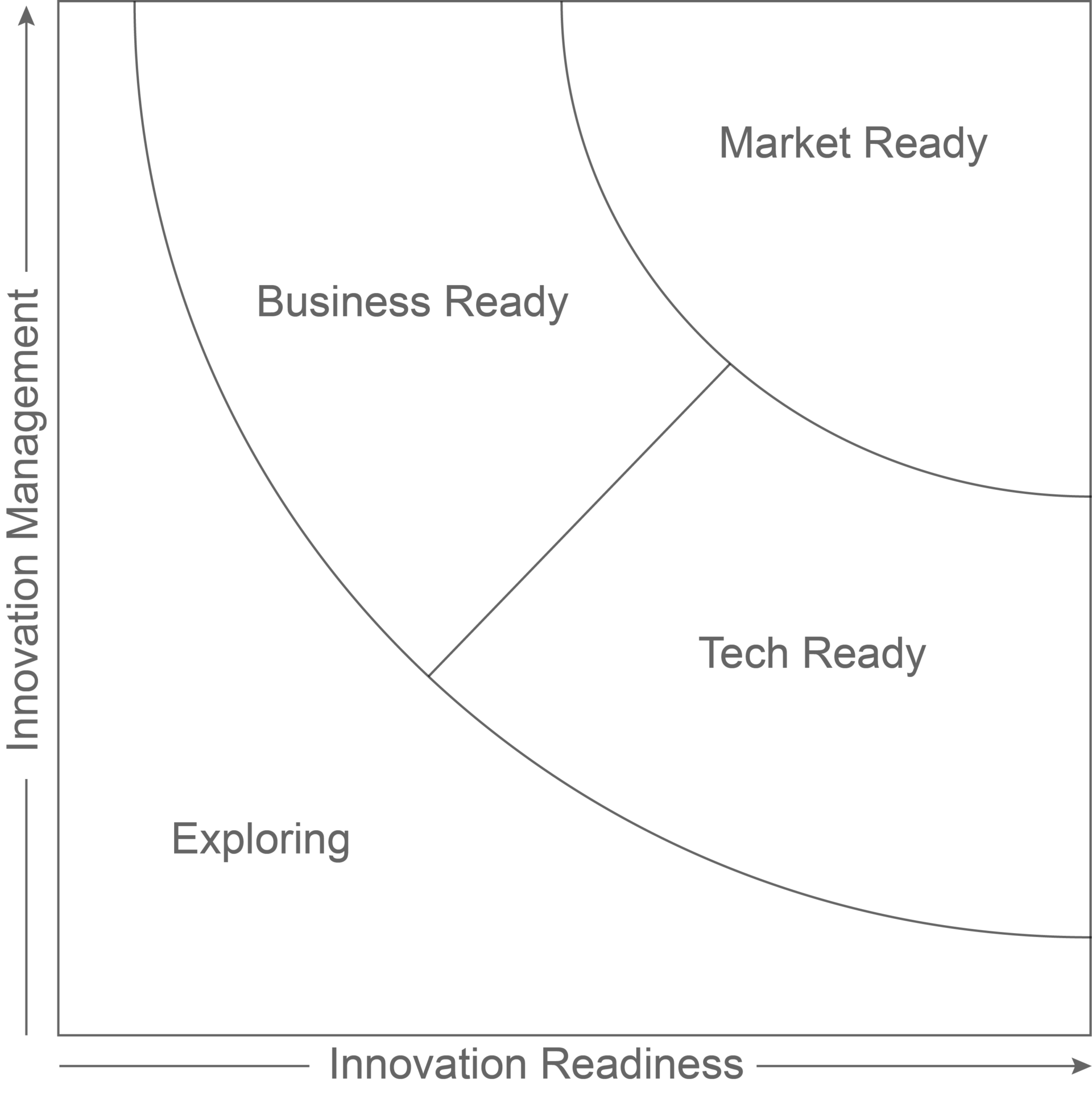
Independent experts from the European Commission’s Innovation Radar have reviewed Lifecares technology in the EU funded project FORGETDIABETES. The review concludes that Lifecare’s innovations are “Market Ready” and considered among the top 14% of innovations in scientific projects receiving financial grants from the European Commission.
Lifecare is part of the EU funded research project FORGETDIABETES, where the company in cooperation with German, Italian and French academic institutions aim to develop an implantable artificial bionic pancreas. Lifecares primary tasks in the project is to develop the sensing element – an “implantable intra peritoneal sensor”, as well as an in-vitro test system for the artificial pancreas beening developed, through Lifecares wholly owned subsidiary Lifecare Laboratory (previously Pfützner Science & Health Institute). In 2020 the European Commission granted the project funding of 3,9 million EUR under the Horizon 2020 program.
The European Commission has established the Innovation Radar as an initiative to identify high potential innovations in EU-funded research. One of the goals of the Innovation Radar is to ensure information of EU-funded research projects. The Innovation Radar analyze information and data gathered by independent experts involved in reviewing ongoing projects funded by the EU, including the independent expert’s views on innovations and market potential. The Innovation Radar methodology is explained here: https://www.innoradar.eu/methodology/#maturity-info
Lifecare’s involvement in the project FORGETDIABETES involves two key innovation tasks.
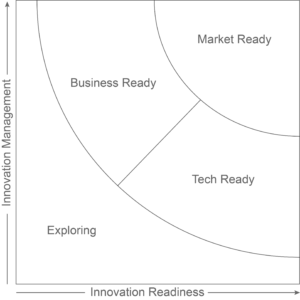 This categorization implies that the two innovations are outperforming in innovations management and readiness, including maturity of technology and commitment from the project consortium leading to the consideration that the innovation is “ready for the market”.
Furthermore, based on signals from the Innovation Radar analyze, Lifecare’s inventions has been categorized in terms of disruptive potential based on the novel indicator system “Market Creation Potential Indicator” (MCPI) developed by the European Commission’s Joint Research Centre.
Lifecares inventions have been categorized with a “HIGH” market potential, the second highest category placing both Lifecare inventions among the top 14 % of all EU-funded innovations. As a matter of fact, 65 % of innovations considered by the MCPI system does not show any Market creation potential and hence a top 14% categorization represents very positive consideration and feedback.
Lifecare has been informed of the conclusions from the Innovations Radar for the purpose of ensuring confidentiality related to invention details before the findings and conclusions will be published at https://www.innoradar.eu within the next 30-45 days.
The independently based consideration of the project-customized sensor implies a strong confirmation of the market potential for Lifecares patented sensor technology. When taking the ongoing pilot clinical studies into account, the outlook of Lifecares technology and market potential is even further enhanced. The independent considerations related to the in-vitro test system for dynamic interference testing of glucose sensors confirm the fact that Lifecare Laboratory already offer and have ongoing commercial third-party contract work based on the system for testing of third-party glucose sensors.
This categorization implies that the two innovations are outperforming in innovations management and readiness, including maturity of technology and commitment from the project consortium leading to the consideration that the innovation is “ready for the market”.
Furthermore, based on signals from the Innovation Radar analyze, Lifecare’s inventions has been categorized in terms of disruptive potential based on the novel indicator system “Market Creation Potential Indicator” (MCPI) developed by the European Commission’s Joint Research Centre.
Lifecares inventions have been categorized with a “HIGH” market potential, the second highest category placing both Lifecare inventions among the top 14 % of all EU-funded innovations. As a matter of fact, 65 % of innovations considered by the MCPI system does not show any Market creation potential and hence a top 14% categorization represents very positive consideration and feedback.
Lifecare has been informed of the conclusions from the Innovations Radar for the purpose of ensuring confidentiality related to invention details before the findings and conclusions will be published at https://www.innoradar.eu within the next 30-45 days.
The independently based consideration of the project-customized sensor implies a strong confirmation of the market potential for Lifecares patented sensor technology. When taking the ongoing pilot clinical studies into account, the outlook of Lifecares technology and market potential is even further enhanced. The independent considerations related to the in-vitro test system for dynamic interference testing of glucose sensors confirm the fact that Lifecare Laboratory already offer and have ongoing commercial third-party contract work based on the system for testing of third-party glucose sensors.
- Lifecare AS is responsible for the development and customization of Lifecares proprietary osmotic pressure-based sensor for glucose monitoring in the subcutaneous area, into an “implantable intra peritoneal sensor”. The sensor represents a critical component for the development of an artificial pancreas.
- Lifecare Laboratory GmbH is a project participant - independent of the mother company - responsible for the development of an in-vitro test system for the sensor and an insulin pump as part of the planned artificial pancreas. As part of this task, Lifecare Laboratory have developed an “In-vitro test bench for dynamic interference testing of glucose sensors”.
 This categorization implies that the two innovations are outperforming in innovations management and readiness, including maturity of technology and commitment from the project consortium leading to the consideration that the innovation is “ready for the market”.
Furthermore, based on signals from the Innovation Radar analyze, Lifecare’s inventions has been categorized in terms of disruptive potential based on the novel indicator system “Market Creation Potential Indicator” (MCPI) developed by the European Commission’s Joint Research Centre.
Lifecares inventions have been categorized with a “HIGH” market potential, the second highest category placing both Lifecare inventions among the top 14 % of all EU-funded innovations. As a matter of fact, 65 % of innovations considered by the MCPI system does not show any Market creation potential and hence a top 14% categorization represents very positive consideration and feedback.
Lifecare has been informed of the conclusions from the Innovations Radar for the purpose of ensuring confidentiality related to invention details before the findings and conclusions will be published at https://www.innoradar.eu within the next 30-45 days.
The independently based consideration of the project-customized sensor implies a strong confirmation of the market potential for Lifecares patented sensor technology. When taking the ongoing pilot clinical studies into account, the outlook of Lifecares technology and market potential is even further enhanced. The independent considerations related to the in-vitro test system for dynamic interference testing of glucose sensors confirm the fact that Lifecare Laboratory already offer and have ongoing commercial third-party contract work based on the system for testing of third-party glucose sensors.
This categorization implies that the two innovations are outperforming in innovations management and readiness, including maturity of technology and commitment from the project consortium leading to the consideration that the innovation is “ready for the market”.
Furthermore, based on signals from the Innovation Radar analyze, Lifecare’s inventions has been categorized in terms of disruptive potential based on the novel indicator system “Market Creation Potential Indicator” (MCPI) developed by the European Commission’s Joint Research Centre.
Lifecares inventions have been categorized with a “HIGH” market potential, the second highest category placing both Lifecare inventions among the top 14 % of all EU-funded innovations. As a matter of fact, 65 % of innovations considered by the MCPI system does not show any Market creation potential and hence a top 14% categorization represents very positive consideration and feedback.
Lifecare has been informed of the conclusions from the Innovations Radar for the purpose of ensuring confidentiality related to invention details before the findings and conclusions will be published at https://www.innoradar.eu within the next 30-45 days.
The independently based consideration of the project-customized sensor implies a strong confirmation of the market potential for Lifecares patented sensor technology. When taking the ongoing pilot clinical studies into account, the outlook of Lifecares technology and market potential is even further enhanced. The independent considerations related to the in-vitro test system for dynamic interference testing of glucose sensors confirm the fact that Lifecare Laboratory already offer and have ongoing commercial third-party contract work based on the system for testing of third-party glucose sensors.